
RELATED
 Sodium Hydrosulfide (NaSH)2024-06-03
Sodium Hydrosulfide (NaSH)2024-06-03 Is calcium chloride a precipitate? What are the uses of calcium chloride?2025-06-27
Is calcium chloride a precipitate? What are the uses of calcium chloride?2025-06-27 Analysis of several common hard carbon biomass precursors2025-06-19
Analysis of several common hard carbon biomass precursors2025-06-19 The Application of Thiourea in Gold Extraction from Gold Ore2025-06-13
The Application of Thiourea in Gold Extraction from Gold Ore2025-06-13 Wishing you peace and wellness on the Dragon Boat Festival.2025-05-30
Wishing you peace and wellness on the Dragon Boat Festival.2025-05-30
MESSAGE
Sodium sulfide
Sodium sulfide, also known as “alkali sulfide”, is the sodium salt of hydrosulfuric acid, molecular formula Na2S, commonly known as “stinking alkali”. Pure alkali sulfide is easily deliquescent white powder, there are also flakes and lumps. Very soluble in water, slightly soluble in alcohol, insoluble in ether, slightly corrosive to most metals. Reacts with acids and emits toxic and flammable hydrogen sulfide gas. Swallowing or inhaling its dust is harmful to human body, corrosive to skin, eyes and mucous membranes. Relative density: 2.427(16℃/4℃), melting point: 50℃; it should be stored in ventilated, dry place, away from light, containers should be sealed to prevent moisture deterioration. Keep it away from heat source and living area, and isolate it from explosives, acids and oxidizers. Wear protective gears and rubber gloves when loading and unloading. Fire-fighting can be done with water or sand. This product can be used to make sodium sulfide by mixing sodium sulfate and carbon and burning to reduce sodium sulfate. Mainly used in the preparation of sulfide dyes and as a reducing agent in organic chemical industry. When the human body is poisoned, it should be quickly moved to fresh air, and artificial respiration should be carried out if necessary. Skin injuries should be washed with water and dilute acetic acid. If this product contains less than 30% water of crystallization, it should be classified as 4.2 flammable substances. UNNo.: 1849/8227/8-08/225, domestic product number: 82011, corrosive.
Applications[2]
1. Application of sodium sulfide in metallurgy
(1) Rare earth leachate decontamination in the treatment of weathered crust leaching type rare earth ore, when a strong electrolyte solution is used to leach the leach, the rare earth leachate obtained often contains a large number of impurity ions, such as Al3+, Fe3+, Ca2+, Mg2+, Cu2+ and so on. When utilizing the oxalic acid precipitation process, these impurities will inevitably form oxalate precipitates transferred to the rare earth product, affecting product purity. Moreover, in order to avoid emulsification in the subsequent extraction process, the impurity ions in the feed solution must first be removed. The solubility product constants of several metal sulfide precipitates are shown in the attached table. When Na2S is added to the rare earth leachate, the heavy metal ions Cu2+, Pb2+, Zn2+, etc. in the solution can be effectively removed. Studies have shown that controlling the pH at about 5, adding Na2S to the rare earth leachate to remove impurities, not only the removal of impurities is effective, and no loss of rare earth.
(2) Treatment of mercury-containing wastewater mercury-containing wastewater on the environment as well as human health are extremely harmful, in the alkali industry, the content of mercury in the discharge wastewater is generally high, more than the international (0.05mg / L). In a weakly attenuating (pH 8-11) solution, mercury ions can generate water-insoluble precipitates with sodium sulfide, and as can be seen from the attached table, the solubility product of HgS is very small (Ksp=1.6×10-52). Through the study, it was determined that the treatment effect at a certain amount of Na2S and controlling the pH value of 9-10 can reduce the Hg2+ in the wastewater to below the national standard (0.05mg/L). In addition, by adding FeSO4 in the water to generate Fe (OH) 2 and Fe (OH) 3 colloids, these colloids can not only adsorb mercury ions, and can trap and coat the suspended HgS solid particles, play a good coagulation and precipitation. Slag is not easy to secondary pollution, easy to dispose of.
(3) the use of Na2S in addition to arsenic arsenic generally exists in the form of sulfide in minerals. In the process of pyrometallurgical smelting, most of the arsenic volatilization into the flue gas and soot, especially the low concentration of SO2 direct emissions will pollute the environment. Therefore, arsenic removal should be carried out before subsequent treatment or evacuation of flue gas. Na2S solution was used to absorb SO2 flue gas, so that As3+ and S2- generate As2S3 precipitation (Ksp=2.1×10-22), and at higher pH (pH>8), As2S3 can be dissolved to generate As3S3-6 or AsS2-3, and at lower pH, the solution produces H2S gas. The study of Yin Aijun et al [4] showed that when controlling the solution pH in the range of 2.0-5.5, reaction time of 50 min, reaction temperature of 30-50 ℃, as well as the addition of flocculants under the conditions of the better removal of arsenic, the rate of arsenic removal of up to 90% or more. In the production of pharmaceutical silica, in order to reduce the production of raw materials in concentrated sulfuric acid impurity arsenic content, to the concentrated sulfuric acid by adding sodium sulfide, so that As3 + As2S3 precipitation removed. Production practice shows that: sodium sulfide in addition to arsenic not only fast reaction speed, and in addition to arsenic thoroughly. After removing arsenic, the arsenic content in sulfuric acid reaches 0.5×10-6 or less, and the arsenic content of silica produced by this raw material is ≤0.0003%, which is in full compliance with the provisions of the U.S. Pharmacopoeia.
4)Application of Na2S in electroplating
Na2S in electroplating as a brightener: sodium sulfide dissolved in water ionized into positively charged sodium ions (Na+) and negatively charged sulfur ions (S2-), in the electroplating process, the presence of S2- in the electrolyte can promote cathodic polarization, in the same current, so that the cathodic reaction speed up. The deposition speed is also accelerated, the deep plating ability increases, the plating layer is refined, and the surface of the plated parts becomes brighter accordingly.
Sodium sulfide in the electrolyte to remove impurities: in the electroplating production process, the impurities in the raw materials will be brought into the plating solution more or less. Under the action of electrode, these impurities undergo different reactions, and the impurities with lower potential will be deposited on the surface of plated parts together with Zn2+, which will affect the quality of plated layer. After adding sodium sulfide, S2- in sodium sulfide can generate precipitates with metal impurity ions, avoiding the participation of impurities in electrochemical reactions and making the plating layer bright.
(5) The use of Na2S solution for flue gas desulfurization At present, the recovery of SO2 in the flue gas is mainly to transform SO2 into H2SO4, liquid SO2 and monomolecular sulfur. Since monomass sulfur is easy to load and unload and transport, it is also an ideal recovery product. A new process to reduce SO2 to monosulfur using H2S produced from Na2S solution as a reducing agent. This process is simple and does not require the consumption of expensive reductants such as natural gas and low-sulfur coal, as is the case with general production techniques. When the solution pH drops to 8.5-7.5, H2S is produced by absorbing SO2 with Na2S, and H2S reacts with SO2 in the liquid phase in a wet Claus reaction.
2. Application of sodium sulfide in mineral processing
(1) sodium sulfide as inhibitor sodium sulfide inhibition of sulfide ore is generally believed to be mainly two aspects, one is the hydrolysis of Na2S to produce HS-, HS-excluding sulfide mineral surface adsorption of yellow medicine, while itself adsorbed on the surface of the mineral to increase the hydrophilicity of the mineral surface; on the other hand, it is believed that the inhibitory effect of Na2S is not only caused by the adsorption of HS- on the surface of the minerals, but also should be associated with the Na2S in the On the other hand, it is believed that the inhibitory effect of Na2S is not only caused by the adsorption of HS- on the mineral surface, but also related to the ionization of Na2S in aqueous solution to generate S2-. For example, when Na2S and yellow medicine are added to galena, the following equilibrium exists
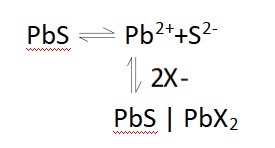
Due to the large solubility product of PbS and the small solubility product of PbX2, when Na2S is added, the concentration of S2- increases and the equilibrium shifts to the left, which leads to the desorption of the yellow drug attached to the surface of the minerals, and thus the inhibitory effect of Na2S on the surface of the minerals. Utilizing the inhibitory effect of Na2S, the flotation of Ni2S3 can be inhibited by adding Na2S, so that the effective separation of Cu2S and Ni2S3 in high ice nickel can be realized. In some lead-zinc beneficiation plants, due to equipment problems and unreasonable production process, the slag still contains high lead-zinc after flotation. However, due to its surface adsorption of certain flotation chemicals, long-term stockpiling produces serious sludging, causing great difficulty in the re-sorting of lead and zinc in the ore. Utilizing the inhibition effect of Na2S, Na2S can be used as a desorption agent to desorb the yellow drug that has been adsorbed on the surface of the minerals, so that the subsequent flotation operation is easy to carry out. The lead and zinc medium ore stockpiled in Shaanxi Xinhe ore dressing plant was pretreated with sodium sulfide for depharmaceuticalization, and then flotation process was used to obtain lead concentrate containing 63.23% lead and zinc concentrate containing 55.89% zinc (the recovery rate of lead and zinc was up to 60.56% and 85.55%, respectively), which made full use of the secondary mineral resources. In the sorting of copper and zinc sulfide ores, due to the dense symbiosis of minerals, sulfur and secondary copper and other characteristics, making the sorting difficult, this kind of ore in the milling process sphalerite has been activated by Cu2+, and its flotation is close to that of chalcopyrite, which makes it not easy to separate copper and zinc minerals. In the treatment of such ores, by adding Na2S in the milling process, the S2- generated by the hydrolysis of Na2S and some heavy metal ions with activation capacity, such as Cu2+ to generate insoluble sulfide precipitation, to remove the activation of these heavy metal ions. Then by adding inhibitors of zinc and sulfur, the copper concentrate containing 25.10% copper, zinc concentrate containing 41.20% zinc and sulfur concentrate containing 38.96% sulfur are obtained by using butylammonium black powder preferential copper - copper tailing zinc - zinc tailing sulfur process.
(2) Sodium sulfide as activator: flotation studies on rhodochrosite-lignite system showed that in limonite amine flotation, amine can be adsorbed on the mineral surface by electrostatic force only at lower pH. However, after the addition of Na2S, which caused the formation of FeS film on the surface of limonite, amine flotation of limonite can be performed at high pH with FeS reagent particles because the FeS film can increase the adsorption of molecular state amines at higher pH. In addition, Na2S can be used as a flotation activator for copper oxide minerals. When the appropriate amount of Na2S is added to the flotation solution, the dissociated S2- and the oxidized mineral surface lattice anions undergo a replacement reaction, which generates a sulfide film on the surface of the copper oxide minerals, thus facilitating the adsorption of the yellow drug type of traps. However, the copper sulfide film generated on the surface of the oxidized copper mineral is not very solid, and it is easy to fall off when stirring strongly. In the treatment of Hubei Daye stone mouth copper ore (malachite-based copper-containing minerals), the use of multi-stage addition of Na2S, multi-point out of the concentrate flotation method, reducing the circulation of the middle ore, copper concentrate grade than the production process increased by 2.1%, copper, gold recovery increased by 25.98% and 10.81%. Na2S can also be used for pyrite flotation inhibited by high alkaline lime system Activator. In the high alkali system, the surface of pyrite is covered with hydrophilic calcium film (Ca(OH)2, CaSO4), so that its flotation is inhibited. It was shown that after the addition of Na2S, the hydrolyzed HS- ions could crowd out Ca(OH)2, CaSO4 and Fe(OH)3 covering the pyrite surface on the one hand, and at the same time they themselves could be adsorbed on the pyrite surface. As pyrite has the ability to transfer electrons, when the interfacial potential of pyrite is greater than EHS/S0, HS- loses electrons on the surface of pyrite to generate hydrophobic elemental sulfur. The generated elemental sulfur is wrapped around the mineral surface, thus making it activated and easy to flotation.
(3) Sodium sulfide as an induced flotation agent for gold and silver minerals: Since the trapless flotation of gold ore makes full use of electrochemical principles and the electronic differences between the surfaces of sulfide and gold and silver minerals, trapless flotation has a higher selectivity and a simpler pharmaceutical system. In addition, it eliminates the non-selective adsorption which is difficult to control in the flotation of yellow drug type of collector, and solves the problem of de-drugging before cyanide leaching of gold and the problem of collector film blocking the leaching of gold, so there are more researches on the flotation of gold-silver minerals without rechargeable collector in recent years. Gold and sulfide minerals in gold and silver ores are often symbiotic, especially gold and pyrite have a close dependence. Due to the pyrite surface has semiconductor nature and certain electron transport ability, and, by comparing the surface electrostatic potential of pyrite with HS-/S0 electric pair EHS-/S0, the surface electrostatic potential of pyrite is always higher than that of EHS-/S0 when the pH of the slurry is in the range of 8 to 13. Therefore, the ionized HS- and S2- of Na2S in the slurry are discharged on the surface of pyrite to generate elemental sulfur .
Preparation [3]
A process for the preparation of high purity sodium sulfide, comprising the following steps:
(1), weighing raw coal and sodium sulfate in the ratio of 1:3-4, and placing the weighed raw coal and sodium sulfate in a ball mill for grinding;
(2), place the powder material obtained in step (1) in a calcining furnace for calcination treatment, control the calcination temperature to be 1000°C-1100°C, and control the calcination time to be 30-50min, both obtaining the sodium sulfide semi-finished product;
(3), the sodium sulfide semi-finished product made in step (2) is diluted with water to form a sodium sulfide solution, and filtered and clarified for 2-3 times;
(4), the sodium sulfide solution treated in step (3) is beaten to the iron remover, adding 0.1-0.3% wt of ferric polysulfate to make the various precipitated substances generated settle, stirring for 20-30min, and filtering at a temperature of 70-80°C, to obtain a sodium sulfide solution with an iron content of less than 10ppm;
(5), the solution of step (4) is evaporated and concentrated to a content of 60%, and the preparation is obtained as a sodium sulfide product with an iron content of 10 ppm or less;
(6), add manganese metal powder and/or manganese-based alloy powder to the zinc sulfate solution at a temperature of 50-60 ℃, and pH>4.5, stirring reaction, after the completion of the reaction, filtration and separation, followed by evaporation and concentration of crystals, to obtain high-purity zinc sulfate, will be prepared high-purity zinc sulfate according to the amount of zinc sulfate added to each cubic filtrate 5-8 kg amount of zinc sulfate weighed, each cubic filtrate corresponds to the preparation of 1 cubic solution of zinc sulfate; Add 140kg of barium sulfide per cubic meter of water, and take the clear liquid for spare; add the above two prepared solutions into the sodium sulfide filtrate at the same time, stir, and control the reaction temperature above 80-90℃, precipitate, remove impurities and clarify, and then send it to evaporation process, and then get the high purity sodium sulfide through evaporation and concentration.
Please give us a message





